-
Synergistic Anion-(pi)n-pi Catalysis on pi-Stacked Foldamers
A.-B. Bornhof, A. Bauzá, A. Aster, M. Pupier, A. Frontera, E. Vauthey, N. Sakai and S. Matile
Journal of the American Chemical Society, 140 (14) (2018), p4884-4892


DOI:10.1021/jacs.8b00809 | Abstract | Article HTML | Article PDF
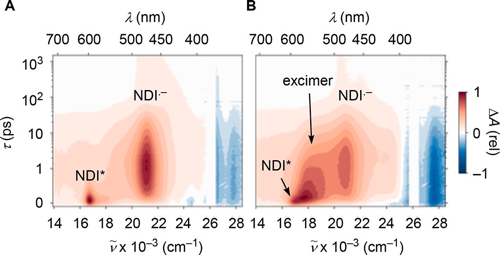
In this report, we demonstrate that synergistic effects between π–π stacking and anion−π interactions in π-stacked foldamers provide access to unprecedented catalytic activity. To elaborate on anion–(π)n–π catalysis, we have designed, synthesized and evaluated a series of novel covalent oligomers with up to four face-to-face stacked naphthalenediimides (NDIs). NMR analysis including DOSY confirms folding into π stacks, cyclic voltammetry, steady-state and transient absorption spectroscopy the electronic communication within the π stacks. Catalytic activity, assessed by chemoselective catalysis of the intrinsically disfavored but biologically relevant addition reaction of malonate half thioesters to enolate acceptors, increases linearly with the length of the stacks to reach values that are otherwise beyond reach. This linear increase violates the sublinear power laws of oligomer chemistry. The comparison of catalytic activity with ratiometric changes in absorption and decreasing energy of the LUMO thus results in superlinearity, that is synergistic amplification of anion−π catalysis by remote control over the entire stack. In computational models, increasing length of the π-stacked foldamers correlates sublinearly with changes in surface potentials, chloride binding energies, and the distances between chloride and π surface and within the π stack. Computational evidence is presented that the selective acceleration of disfavored but relevant enolate chemistry by anion−π catalysis indeed originates from the discrimination of planar and bent tautomers with delocalized and localized charges, respectively, on π-acidic surfaces. Computed binding energies of keto and enol intermediates of the addition reaction as well as their difference increase with increasing length of the π stack and thus reflect experimental trends correctly. These results demonstrate that anion–(π)n–π interactions exist and matter, ready for use as a unique new tool in catalysis and beyond.

